Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common B-cell malignancy worldwide. A sizable fraction of these patients ended up succumbing to this fatal disease, so the standard of care after the second-line chemotherapy and/or autologous stem cell transplants (ASCT) remains to be improved.
Polatuzumab vedotin-piiq (PoV), an anti-CD79b antibody-drug conjugate, gained accelerated approval by the U.S. Food and Drug Administration in June, 2019. The phase II randomized controlled study (GO29365) showed PoV in combination with bendamustine and rituximab (BR) conferred an additional 22% complete response rate for relapsed/refractory DLBCL, compared with BR alone (40% vs. 18%) . Interestingly, how PoV fares when combined with other salvage therapy remains as an open question.
Method
DLBCL patients who developed progressive diseases after at least 2 prior lines of therapy including R-CHOP and not previously treated with BR were enrolled into this study between Nov. 2018 and Apr. 2020 at the National Taiwan University Hospital. PoV was given at 1.8 mg/kg in combination with salvage chemotherapies based on clinicians' choices. The compassionate use of PoV was approved by the ethics committee, and clinical data were retrospectively analyzed.
Results
Thirty-two patients were enrolled in this study. According to the Hans' criteria of immunohistochemical subtypes, 13 patients were germinal center B cell-like (GCB), 17 were non-GCB, and 2 patients were unclassifiable. The median age was 62.5 years (range 20-82). At diagnosis, 26 (81%) patients had stage III or IV diseases, 22 (68.8%) had extranodal involvement, and 24 (75%) had the IPI scores equaled to 3 or higher. The median of failed prior lines of treatment was 4 (range 2-11), including 7 (21.9%) relapsing from ASCT and 16 (50%) were of primary refractory disease. Regarding combination therapies, 20 (62.5%) received BR, 5 (15.6%) had rituximab, 5 (15.6%) had rituximab/carmustine-containing regimens, and rituximab/gemcitabine-based ones were given to 4 (12.5%) patients. The median of cycles of PoV was 4 (range 1-7). Notably, the combinatory use of PoV and salvage chemotherapy helped transition 5 patients to ASCT and 4 to allogeneic SCT.
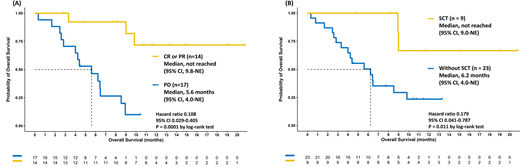
The median overall survival (OS) was 8.9 months (95% confidence interval 6.2-not estimable) with the median follow-up of 11.4 months (range 0.3-20.7). The overall response rate (ORR) was 45.2%, including 29.1% complete response (CR) and 16.1% partial response (PR). The patients achieving CR or PR after PoV had better overall survival (OS) than those who did not (median OS: not reached (NR) vs. 5.6 months, p=0.0001, Figure A). The ORR for patients failing prior 2, 3, 4, and ≥5 lines of treatment were 50% (3/6), 40% (2/5), 50% (4/8), and 42% (5/12), respectively. The patients failing prior 4 lines of treatment had better OS than those with failed prior 5 or more lines (NR vs. 6.47 months). Non-GCB patients had better ORR (69.2% vs. 30.8%) and OS (NR vs. 6.23 months) than GCB ones did. Intriguingly, the patients received SCT had better OS compared with the others (NR vs. 6.23 months, p=0.011, Figure B).
As for grade 3 & 4 hematological adverse effects, 16 (50%) patients developed anemia, 20 (62.5%) had thrombocytopenia, and 18 (56.3%) were neutropenic. Grade 5 toxicities included pneumonia (2 patients, 6.3%), fungemia (1, 3.1%), intracranial hemorrhage (1, 3.1%), and other infections (2, 6.3%).
Conclusion
As the third-line or above treatment for DLBCL in this study, the efficacy and safety associated with PoV-based therapies not only were encouraging but also helped bridge to transplants. The application of this monoclonal antibody-drug conjugate in future clinical trials for DLBCL is expected.
Tsai:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Chugai: Honoraria; Harvester: Honoraria; Janssen: Honoraria; Kirin: Honoraria; Takeda: Honoraria; BMS: Honoraria; Astellas: Honoraria; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees. Ko:Roche: Honoraria. Cheng:Roche: Honoraria. Huang:MundiPharma: Honoraria; Chugai: Honoraria; Roche: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Janssen: Honoraria; Takeda: Honoraria; Bristol Meyer & Squibb: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal